EU Reference Labs for IVD’s – explained
In vitro diagnostic medical devices (IVDs) play a vital role in providing essential information for the diagnosis, monitoring, and treatment of diseases. IVDs must comply with the In Vitro Diagnostic Medical Device Regulation (IVDR – Regulation (EU) 2017/746) to be placed on the European Union (EU) market.
The IVDR introduced the concept of EU Reference Laboratories (EURLs) for IVDs. EURLs are specialised laboratories designated by the European Commission, to provide scientific and technical expertise for specific types of high-risk IVDs. The designation and responsibilities of EURLs are outlined in Article 100 of the IVDR and more details are laid out in Commission Implementing Regulation (EU) 2022/944.

Designation & Responsibilities of EURLs
For a laboratory to be designated as an EURL by the European Commission, it must:
- Demonstrate appropriate expertise and experience in the relevant field
- Have adequate staff with appropriate qualifications
- Possess necessary equipment and reference materials
- Show impartiality and independence from commercial interests
- Comply with ISO/IEC 17025 and other relevant standards
- Have appropriate quality management systems in place
Once designated, EURLs’ tasks include the following:
- Performance Verification – EURLs verify the performance claims of class D IVDs by conducting laboratory tests on samples provided by manufacturers i.e., EURLs carry out tests on each manufactured batch of devices.
- Common Specifications Development – EURLs contribute to the development of common specifications and technical guidance for specific types of devices.
- Technical Assistance – EURLs provide scientific advice and technical assistance to national authorities, notified bodies and manufacturers.
- Reference Materials Management – EURLs establish, manage, and make available reference materials and methods for high-risk IVDs.
- Coordination Role – EURLs coordinate a network of national reference laboratories to harmonize testing methodologies and practices across the EU.
- Research and Innovation – EURLs contribute to research and innovation in their specific areas of expertise.

Types of IVDs Subject to EURL Assessment
The EURLs verify the performance claims and carry out batch testing on high-risk (Class D) IVDs:
- Devices which detect the presence of / exposure to, a transmissible agent in blood / blood components / cells tissues / organs / any of their derivatives, to assess their suitability for transfusion / transplantation / cell administration.
- Devices which detect the presence of / exposure to, a transmissible agent that causes a life-threatening disease with high propagation risk.
- Devices which determine the infectious load of a life-threatening disease, where monitoring is critical in the process of patient management.
- Devices for blood grouping, which determine any of the following markers:
- ABO system [A (ABO1), B (ABO2), AB (ABO3)]
- Rhesus system [RH1 (D), RHW1, RH2 (C), RH3 (E), RH4 (c), RH5 (e)]
- Kell system [KEL1 (K)]
- Kidd system [JK1 (Jka), JK2 (Jkb)]
- Duffy system [FY1 (Fya), FY2 (Fyb)]
Impact on the Industry
EURLs for IVDs have an impact on both IVD manufacturers and notified bodies.
Manufacturers of class D IVDs must submit samples of their device to an appropriately designated EURL for the EURL to verify performance claims and compliance to any common specifications. Manufacturers must then incorporate any feedback into their technical documentation. Prior to release of a batch of any Class D IVDs, manufacturers must wait for EURLs to confirm they have completed their tests.
Notified bodies will need to consult with EURLs when assessing certain high-risk IVDs and factor in EURL’s opinions into their assessment decisions. Notified bodies will also have to maintain communication with EURLs for post-market surveillance.

Implementation Challenges
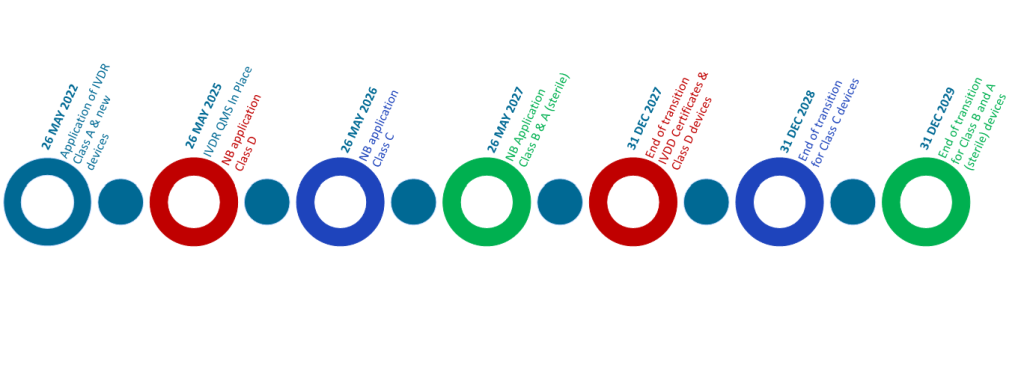
Although the IVDR became fully applicable on 26 May 2022, implementation of the regulation has not necessarily been smooth. The timelines to which certain devices must be compliant to the IVDR have been extended more than once and establishing a EURL network has been challenging. Resources have been restrained, as building the necessary infrastructure and hiring the appropriate expertise has required significant investment and coordination.
As a result, the European Commission adopted a phased approach to EURL designation and until EURLs are fully operational for all relevant device categories, interim procedures have been established for the conformity assessment of class D IVDs. MDCG 2021-4 provides guidance on how EURLs should be integrated into the conformity assessment process when designated.
Current State of Play
The European Commission have designated the following 5 EURLs by the implementing act Commission Implementing Regulation (EU) 2023/2713
| Designated EU reference laboratory | Scope of designation Class D devices intended for detection or quantification of markers of: |
| Consortium managed by Servicio Madrileño de Salud (SERMAS), Spain and composed of: Hospital General Universitario Gregorio Marañón, Spain Hospital Universitario la Paz, Spain Hospital Universitario Ramón y Cajal, Spain | Herpesvirus infection Infection with bacterial agents |
| Consulting Químico Sanitario SLU (CQS), Spain | Herpesvirus infection Infection with bacterial agents |
| EU Referenzlabor für In-vitro-Diagnostika am Paul-Ehrlich-Institut (PEI-IVD), Germany | Hepatitis or retrovirus infection Respiratory virus infection |
| Instituto de Salud Carlos III (ISCIII), Spain | Hepatitis or retrovirus infection Herpesvirus infection Infection with bacterial agents |
| RISE Research Institutes of Sweden AB (RISE), Sweden | Respiratory virus infection |
These EURLs took up their tasks in the conformity assessment of devices on 1 October 2024.
In February 2025, the European Commission launched a further call for more applications to be submitted by Member States on behalf of their candidate laboratories for designation of EURLs. This call will be conducted in 2 waves.
The 1st wave is for the following categories of class D devices:
- detection or quantification of markers of arbovirus infection
- detection or quantification of markers of parasite infection
- detection of blood grouping markers
Laboratories must submit applications to their Member State by 15 April 2025 (note: this date is indicative and laboratories should check with their specific Member State).
Member States must forward applications to the Commission by 6 June 2025.
The 2nd wave is for any of the following 8 categories of class D devices:
- detection or quantification of markers of hepatitis or retrovirus infection
- detection or quantification of markers of herpesvirus infection
- detection or quantification of markers of infection with bacterial agents
- detection or quantification of markers of arbovirus infection
- detection or quantification of markers of respiratory virus infection
- detection or quantification of markers of infection with haemorrhagic fever viruses or other biosafety level 4 viruses
- detection or quantification of markers of parasite infection
- detection of blood grouping markers
Laboratories must submit applications to their Member State by 15 January 2026 (note: this date is indicative and laboratories should check with their specific Member State).
Member States must forward applications to the Commission by 15 April 2026.
Conclusion
EURLs play a critical role in ensuring certain Class D IVDs are safe and effective. Although there are interim procedures in place for the conformity assessment of Class D IVDs, the IVD industry will benefit from the certainty of having enough designated EURLs to cover all categories of Class D devices.
Let’s hope this new call for further applications for designation of EURLs will provide additional resources the EU IVD industry desperately needs.
If you’re looking for a regulatory partner to offer compliance advise and support, get in touch with IVDeology today to begin your new partnership or email us at [email protected]
Exploring the EU COMBINE Programme
Understanding and Bridging the Gap in Clinical Research The European Union’s (EU) healthcare l…
What Are EU Reference Labs for IVDs?
EU Reference Labs for IVD’s – explained In vitro diagnostic medical devices (IVDs) play …
A review of the MHRA Fee Consultation
Executive Summary The MHRA (Medicines and Healthcare products Regulatory Agency) held a public consu…
eQMS is Like a Chocolate Selection box: Choosing the Right Quality Management System
Choosing an electronic Quality Management System (eQMS) might not sound like it has much in common w…
Understanding Companion Diagnostics: A Key to Personalised Medicine
The first companion diagnostic (CDx) was approved by the US Food and Drug Administration (FDA) in 19…
IVDeology now offering QMS Hosting Services!
One of the biggest challenges we see is the adoption of an effective Quality Management System (QMS)…